testing the hardness of water chemistry|check my water hardness : custom Try this practical with your students to measure the hardness of water samples and investigate the effect of boiling. Includes kit list and safety instructions. WEB2023-11-10 13:22:32.163000 Ivana >>121880044 I had the same issue with voting - if I clicked the actual post and voted within that it worked for me, but it didnt as long as I stayed looking at the 'patreon feed' if that makes sense. All the same, I .
{plog:ftitle_list}
Resultado da için Windows oyunlarını ücretsiz indirin: Brawl Stars (Gameloop), Pes 2013, Yandere Simulator. Onları ücretsiz ve virüssüz olarak indirin.
Chemistry investigatory HARDNESS OF WATER. This document describes a student's chemistry investigatory project to test drinking water samples for hardness, iron, fluoride, and chloride. The student outlines .Try this practical with your students to measure the hardness of water samples and investigate the effect of boiling. Includes kit list and safety instructions.3) EDTA titration can be used to determine water hardness by chelating the Ca2+ and Mg2+ ions until the indicator changes color, indicating the endpoint of the titration reaction. 1) Hard water is caused by high mineral content, mainly .Water hardness can be readily determined by titration with the chelating agent EDTA (ethylenediaminetetraacetic acid). This reagent is a weak acid that can lose four protons on .
Key Concepts. Calcium and magnesium salts dissolved in water cause water hardness. Water hardness can be measured using a titration with ethylenediaminetetraacetic acid (EDTA). The ionised form of EDTA is .
The simple definition of water hardness is the amount of dissolved calcium and magnesium in the water. Hard water is high in dissolved minerals, largely calcium and .In this experiment, you will determine the “hardness” of a water sample by complexometric titration. Learning Objectives Apply concepts of complexation chemistry to determine the .You will use EDTA complexometric titration to determine the hardness of a sample of water brought from your home. Both the total hardness and the individual calcium and magnesium .
Testing for Water Hardness Background Info: Hard water has a lot of minerals (Calcium and Magnesium mainly) dissolved in it. Hard water does not mix well with soaps. Because of this, .
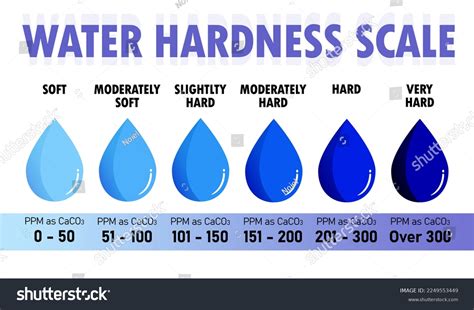
This is the classic method to determine the total water hardness over a titration with EDTA solution.Patreon: https://www.patreon.com/randomexperimentsintern. Alkalinity or Acid Neutralizing Capacity (ANC) Purpose: The purpose of this assignment is to introduce concepts of titrimetry as they pertain to the determination of ANC and water hardness of water samples. Learning . Chemical tests for water Chemical tests for water. The presence of water is commonly tested for using anhydrous cobalt(II) chloride or anhydrous copper(II) sulfate. Cobalt(II) chloride. Anhydrous cobalt(II) chloride, CoCl 2, is blue. Hydrated cobalt(II) chloride, CoCl 2 •6H 2 O is pink. So, anhydrous cobalt(II) chloride can be used to test .Moderately hard water: 50-150 mg/ltr; Hard water: 150-300 mg/ltr; Very hard water:>300 mg/dl; Hard water is not suitable for industrial use. But hard water is usually beneficial for drinking purposes. However, hardness caused by .
Hard water interferes or reduces lathering (the formation of suds or bubbles). Because of hard water, a film can build up on shower doors and walls, or bath tubs, sinks, and faucets. Hair washed with hard water might look less shiny than hair washed with soft water. Clothing fabrics might be dull, gray, and scratchy, and wear out more quickly. Chemical Water Analysis Methods. Chemical analysis is a fundamental part of water quality analysis. It requires testing for different chemical parameters to identify contaminants and assess their levels in water. Some of the commonly tested chemical parameters include ammonia, chloride ion, nitrite, nitrate, phosphate, and water hardness. Schedule for Testing Pool Water for Iron. Once a month. You should test if you notice a brown coloration or have a low pH reading. Testing for Calcium. This is the same as testing for calcium hardness or water hardness. Schedule for Testing Pool Water for Calcium. Once a month. Testing for PhosphatesEstimation of Hardness of Water by EDTA Method 3 In the pH range 8-10, the blue form of the indicator HD2– gives a wine red complex with Mg2+: Mg+2 + HD 2– MgD– + H+ (Blue) (Wine red) Now if EDTA (H2Y 2–) is added to such a solution Mg2+ preferentially complexes with EDTA (since the metal EDTA complex is more stable than the metal-indicator complex) and liberates .
1) Hard water is caused by high mineral content, mainly calcium and magnesium ions, that leach into water supplies. These ions interfere with soap and cause scale buildup. 2) Hardness can be temporary, caused by bicarbonates that can be removed through boiling, or permanent, caused by sulfates and chlorides. 3) EDTA titration can be used to determine water hardness by .
water hardness testing procedure
methods to determine water hardness

There are three different ways you can test your pool water chemistry: using pool test strips, using a pool testing kit, or using an electronic device. Each method has pros and cons. . Most experts recommend using a calcium hardness increaser, such as calcium chloride, if your water’s calcium hardness levels fall below 200 or 150 ppm. If .
This practical guide for beginners explains what these water chemistry terms mean, when you should test for them, and how to raise or lower them if needed. . use a calcium test kit (specifically for fresh water) to determine if you’re lacking that particular mineral. . If you already have hard water coming out of the tap, these .
Water testing is a broad description for various procedures used to analyze water quality. Millions of water quality tests are carried out daily to fulfill regulatory requirements and to maintain safety. . See Bacteriological water analysis and specific tests such as turbidity and hard water. . Reagents are chemical testing compounds that .
A bathtub faucet with built-up calcification from hard water in Southern Arizona.. Hard water is water that has a high mineral content (in contrast with "soft water"). Hard water is formed when water percolates through deposits of limestone, chalk or gypsum, [1] which are largely made up of calcium and magnesium carbonates, bicarbonates and sulfates.. Drinking hard water may .
Abstract This paper presents a test method for determining the total hardness in natural and drinking waters using an indicator solution for test titration. The developed method offers a rapid and efficient procedure for assessing the total hardness across a wide range of water bodies. The relative standard deviation of a single result in determining the overall .Hardness in water is caused by dissolved minerals, primarily divalent cations, including calcium(Ca2+), iron (Fe2+), strontium (Sr2+), zinc (Zn2+) and manganese (Mn2+). . EGTA and EDTA, are used in the test. Chemical Reactions: Total hardness titration. Several solutions including digital titrator cartridges are described in the following .Hardness of water is because of the presence of salts of calcium and magnesium. Know the different Types of Hardness in water and the process to Remove Temporary and Permanent Hardness. . JEE Main 2021 LIVE .How we measure water hardness. Water hardness depends on how many milligrams per litre (mg/L) of calcium carbonate (CaCO 3) it contains. If the concentration is less than 60 mgCaCO 3 /L, it’s considered to be soft. If the .
This water testing kit also includes a circular water treatment chart, helping you adjust the chemical levels if needed. Note that this pool kit has more components than more basic options, and it comes with an informative, . You can easily test the water hardness using test strips or liquid test kits which measure the levels of calcium in the water. The desired range for hot tub water hardness typically falls between 175 and 250 ppm (parts per million). . it is important to take steps to increase it to the recommended range for optimal water chemistry. Low water .
Solution: A properly working water softener will reduce the hardness in boiler feedwater to less than 1ppm. In almost all systems with softener upsets, scale is forming. It will get worse with more hardness and time. The real solution here is to test your softener daily (1-minute test) and ensure there is proper maintenance of the unit over time.Detergents can lather well in the hard water. Detergent is the sodium salt of a long chain benzene sulphonic acid (or the sodium salt of a long chain of alkyl hydrogen sulfate), which repels the Ca 2 + or Mg 2 + ions.. When added to water, they don’t produce insoluble precipitates, and therefore dissolve easily in the hard water. This is the classic method to determine the total water hardness over a titration with EDTA solution.Patreon: https://www.patreon.com/randomexperimentsintern.
What is Hard Water? Hard water is a term that denotes water having a very high mineral content (the term is the opposite of ‘soft water’). As water percolates into deposits of calcareous, gypsum or chalk that are primarily composed of carbonates of magnesium or calcium, bicarbonates and sulphates, hard water is formed. Drinking hard water can have certain benefits to human health.Answer: The primary source of water pollution are mentioned below. Disposal of industrial effluents into water. Improper Sewage Disposal. Oil Spills; Fertilizer Run-Off. Q9. We can remove hardness in temporary water by boiling, but the hardness in permanent hard water cannot be removed by boiling. Give reason.This document describes a chemistry project to test drinking water for hardness, iron, fluoride, and chloride. The project was submitted to the CBSE board under the supervision of Mrs. Manisha Sharma by Shiwan Sharma. The objective was to determine the levels of these ions depending on the water source and study the causes of their presence. The document .

Table 01: Categories of water based on the hardness. EDTA titrimetric method of determining the total hardness of water. Ca 2+ and Mg 2+ ions in the water sample are titrated with Ethylenediaminetetraacetic acid (EDTA) at 10.0 ± 0.1 pH. We use the Eriochrome black T as the indicator here.. First, use the Eriochrome black T indicator will be complexed with Ca 2+ .This initial demonstration produces a range of different samples which students can test for hardness using soap solution, before finally investigating the effect of adding sodium carbonate. Using their results, students can reflect on how effectively each treatment softens hard water. . Environmental chemistry: water. 9.2 Hardness of Water .
Hardness of water also can be tested by a more rapid test strip method. The commercial test strips contain EDTA and an indicator chemical to cause a color change when the calcium and magnesium in water react with the EDTA. In this laboratory exercise, hardness of water is determined by both the EDTA titration method and with Quantab ® test strips.
how to test water hardness at home
how to determine water hardness
WEBLucky 777 Gaming
testing the hardness of water chemistry|check my water hardness